· The atomic mass of an atom is the sum of its protons and neutrons or Z N The strong nuclear force binds protons and neutrons together to form the nucleus of an atom Electrons Electrons are much smaller than protons or neutrons and orbit around them0% Home AQA GCSE Chemistry Topic Questions 1 Atomic Structure & the Periodic Table 11 Simple Model of the AtomMedium View solution Describe Bohr's model of the atom Medium View solution Calculate the energy associated with the first orbit of H e What is the radius of this orbit ?
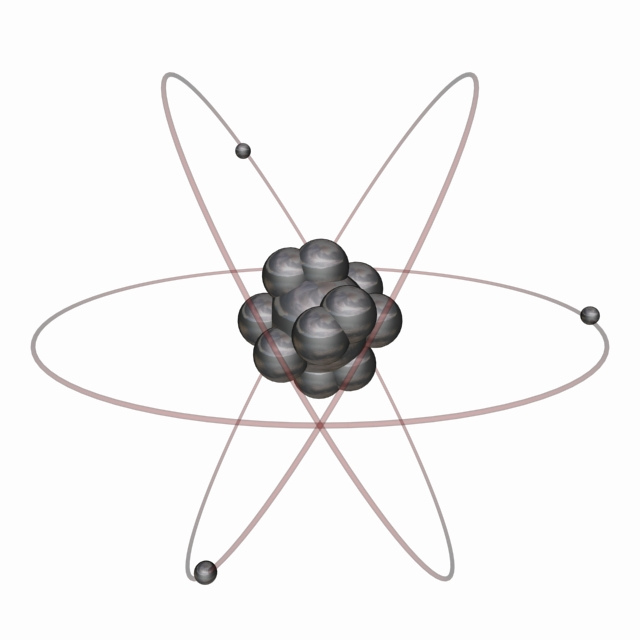
What Atomic Model Is In Use Today Socratic
Model of an atom drawing easy
Model of an atom drawing easy-7 Have students make a simple drawing of their model of the carbon atom 8 Have students refer to the periodic table of elements to find the chemical symbol for carbon, noting that it is element six and that it has six protons Have students use the periodic table and the same candy pieces to draw and label simple models of the hydrogen,The Bohr Model is a modification of an earlier atomic model, the Rutherford Model The Bohr Model has an atom with a positivelycharged nucleus surrounded by negativelycharged electrons that have circular, planetarylike orbits Today, we know that the Bohr Model has some inaccuracies, but it's still used because of its simple approach to



Rutherford Model Wikipedia
Molecular graphics (MG) is the discipline and philosophy of studying molecules and their properties through graphical representation IUPAC limits the definition to representations on a "graphical display device" Ever since Dalton's atoms and Kekulé's benzene, there has been a rich history of handdrawn atoms and molecules, and these representations have had an important influenceHow to Draw an Atom! · An atom model project is a great way to introduce students from elementary school through high school to the 3D concept of the atom, and how the protons and neutrons in the nucleus relate geometrically and in terms of size to the electrons spaced at relatively vast distances from the nucleus
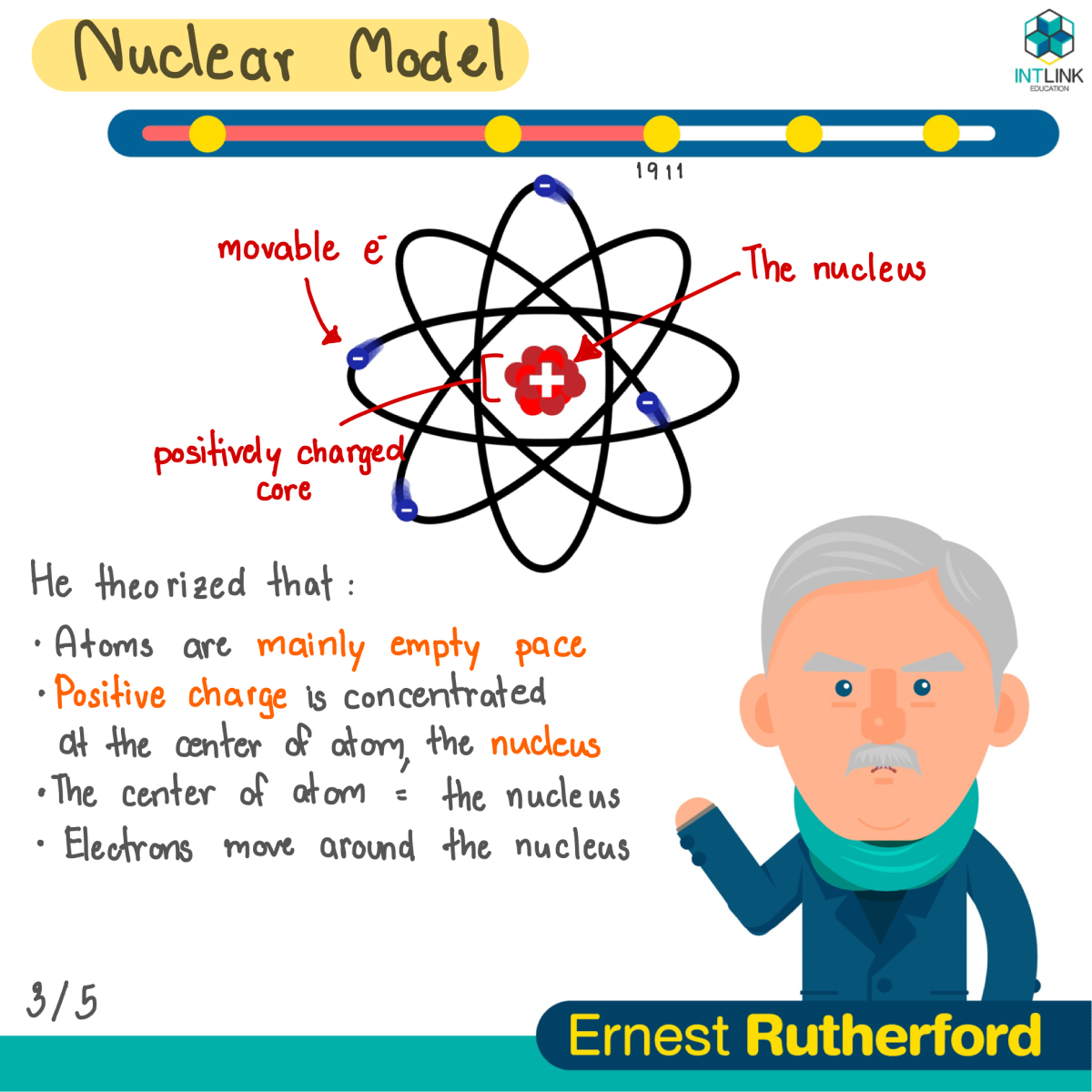
· Electron Shells Electrons orbit around the nucleus in a series of shells Each shell can hold a certain maximum number of electrons (2 for the first shell;Bohr Atomic Model A Danish physicist named Neil Bohr in 1913 proposed the Bohr atomic model He modified the problems and limitations associated with Rutherford's model of an atom Earlier in Rutherford Model, Rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles) · The Bohr model of an atom was able to explain the stability of the atom and also could explain the phenomenon of atomic spectra and ionization of gases James Chadwick and Discovery of Neutrons There were a lot of problems related to the calculation of the mass of the nucleus, although Rutherford's model could explain the scattering of alpha problem
1808 Dalton proposed a modern atomic model based on experimentation not on pure reason •All matter is made of atoms •Atoms of an element are identical •Atoms of different elements are distinctively different •Atoms are rearranged in chemical reactions •Atoms of different elements combine in constant ratios to form compoundsUse the number of protons, neutrons, and electrons to draw a model of the atom, identify the element, and determine the mass and charge Predict how addition or subtraction of a proton, neutron, or electron will change the element, the charge, and the massThis mode is convenient to draw a molecule composed of numerous atoms of the same type Let us draw perfluoroalkane as an example Assume we want CT type carbon atoms, F type fluorine atoms, and 01 charge The atom model is to be saved in hin format Select methane as a primer by clicking the button in the Kernel box



How To Draw The Atomic Structure Of Atoms




Bohr Model Description Development Britannica
· Simple Model Of The Atom Every atom has a central, positively charged nucleus that is very small but massive The nucleus is made up of positive protons and neutral neutrons Electrons revolve around the nucleus in allowed orbits A vast amount of empty space within an atomThe Atom Science >> Chemistry for Kids The atom is the basic building block for all matter in the universe Atoms are extremely small and are made up of a few even smaller particles The basic particles that make up an atom are electrons, protons, and neutrons Atoms fit together with other atoms to make up matterDrawing Bohr Models 1 Determine the number of rings, or energy levels (Look at the period, or row, number) 2 Determine the number of protons, neutrons, and electrons 3 Determine the number of valence electrons (Look at the group, or column, number) 4 Draw the correct number of rings 5 Draw the correct number of electrons on each




How To Draw An Atom Really Easy Drawing Tutorial



How To Draw An Atom Worksheets Teaching Resources Tpt
Bohr model, description of the structure of atoms, especially that of hydrogen, proposed (1913) by the Danish physicist Niels BohrThe Bohr model of the atom, a radical departure from earlier, classical descriptions, was the first that incorporated quantum theory and was the predecessor of wholly quantummechanical models The Bohr model and all of its successors describe theThe first shell is tiny and can only hold 2 electrons all other shells hold 8 electrons (you'll learn that this isn't quite true at Alevel) Let's start by drawing lithium together stepbystep Grab a piece of paper and draw beryllium click on this link to see if you're correct!Introduction to the atom Transcript Learn how atoms are made up of protons, neutrons, and electrons Elements are defined by the atomic number, the number of protons in the nucleus The mass of an atom is determined by the total number of protons and neutrons Created by Sal Khan



Drawing Atoms Montessori Muddle




Basic Chemistry Tutorial 2 Drawing Atoms Sciencemusicvideos
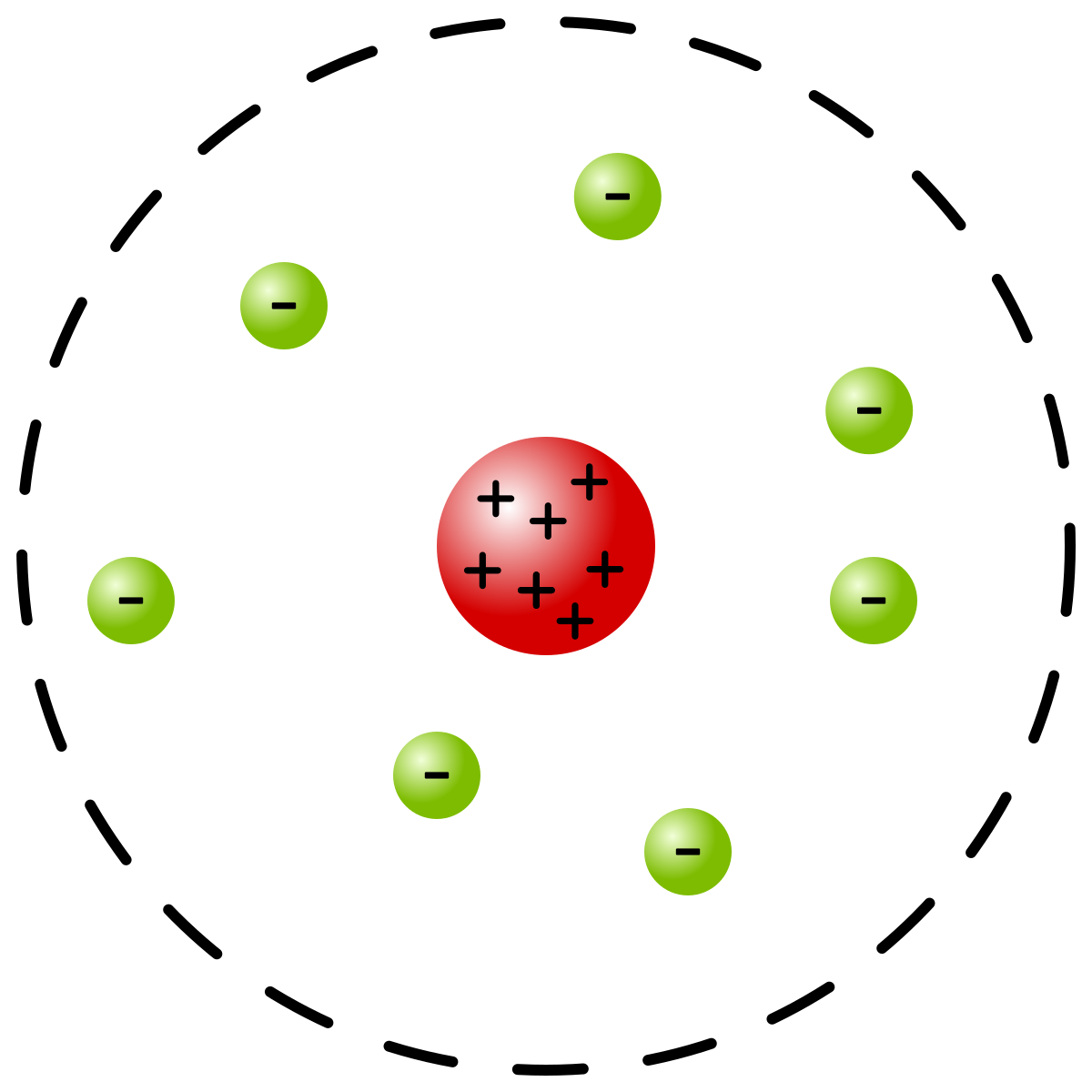
Cardboard large enough to accommodate the model;Bohr atomic model is one of the best way to get somewhat of a visual representation (although incomplete) of atomic structure and understand why atoms behave the way they do This board captures as much details into it as possible, to make it more intuitive For example, the different energy levels are engraved to be stepsIn atomic physics, the Bohr model or Rutherford–Bohr model, presented by Niels Bohr and Ernest Rutherford in 1913, is a system consisting of a small, dense nucleus surrounded by orbiting electrons—similar to the structure of the Solar System, but with attraction provided by electrostatic forces in place of gravityAfter the cubical model (1902), the plum pudding model (1904), the
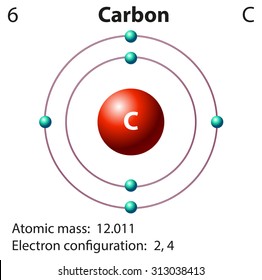



Carbon Atom Model High Res Stock Images Shutterstock



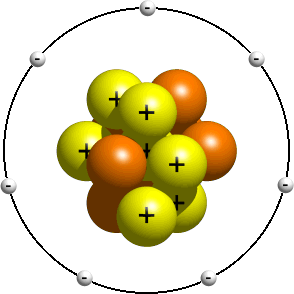
How To Draw An Atom Youtube
· Scale Model of an Atom Student Worksheet Use this worksheet as a template to make your own custom worksheet To create the scale distance mark I measured the scale distance (10 miles) on the Google Map (lower right corner) with a 'Postit' note and recreated that measurement on the worksheet Student Examples · /captionThe image on the left is a basic atom diagram This one shows the protons, neutrons, and electrons of a carbon atom Each is in a group of six That makes the atomDalton's atomic model sets up the building blocks for others to improve on Though some of his conclusions were incorrect, his contributions were vital He defined an atom as the smallest indivisible particle Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time




Labeled Parts Of An Atom Diagram Atom Diagram Atom Worksheets




How To Draw Bohr Diagrams Slideshare
Easy Atomic Models By Lisa B 4 Comments I was We then used their "Think About It" section to add a little fun to our Math Journal by drawing out an atom of lead The counting up to 125 was a lot of work!We collected 36 Atom Drawing paintings in our online museum of paintings PaintingValleycom Atom Drawing For Fre Drawing Atoms Montes The Rags Of Time' J Atom Drawing Physics Atom Drawing Free Do Atom Drawing Cartoon Atom Drawing Illustr Atom Drawing Atomic · How to Draw an Atom!




Introduction To Structure Of Atom Proton Neutron Electron With Examples



Questions And Answers How Do I Make A Model Of An Atom
(PS the link opens in a new page)View Answer Topics Nuclear Chemistry Intro to Chemistry Composition Living by Chemistry Chapter 3 A World of Particles Section 2 Atomic Number and Atomic Mass · Dalton's simple model of an atom persisted until 17, when another British physicist, JJ Thomson, discovered that atoms contained tiny
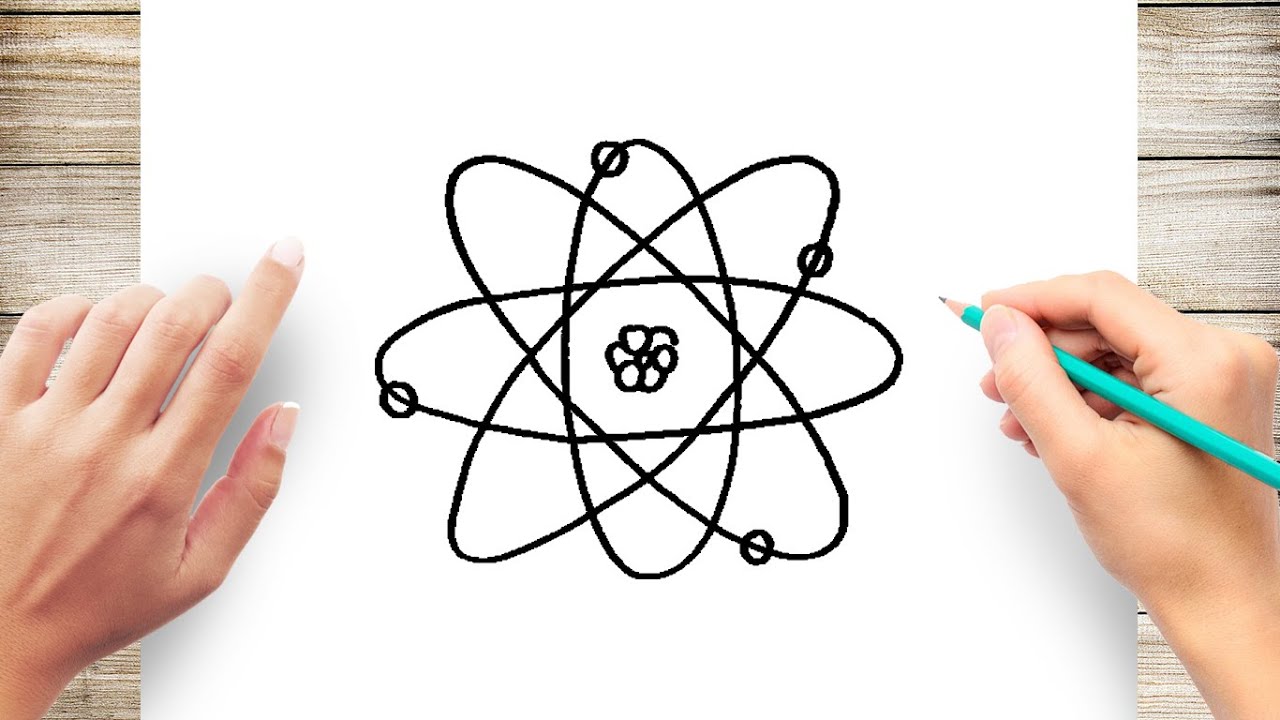



How To Draw An Atom Step By Step Simple And Easy Youtube




Simple Atom Ii Sticker By Kruzzell In 21 Science Stickers Black And White Stickers Aesthetic Stickers
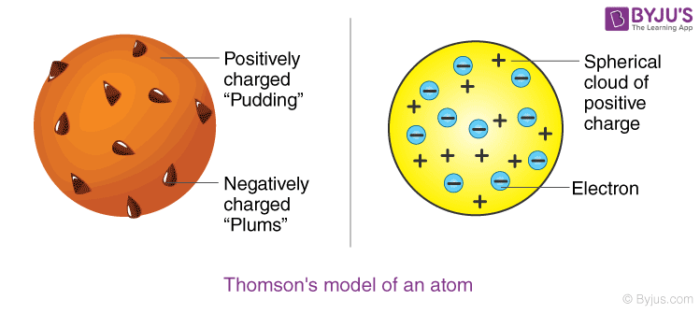
Avogadro Avogadro is a free molecular modeling software for Windows It comes with a simple interface which offers a hasslefree chemical drawing Some commonly used elements (carbon, oxygen, boron, fluorine, phosphorus, etc) are available in its dropdown menu Other elements can be selected from the builtin periodic tableDiscovery, Thomson proposed a new atomic model According to Thomson's model, the atom was like a plum pudding it was mostly a thick, positively charged material, with negative electrons scattered about it like plumsin a pudding In 908, Ernest Rutherford took an extremely thin sheet of gold foil and bombarded it with electronsThe nuclear shell model is a model of the atomic nucleus It uses the Pauli exclusion principle to explain the nucleus structure in terms of energy levels Understand the Shell Model of an Atom with examples, explanations, difference and more at BYJU'S




Rutherford Model Of The Atom Definition Diagram Video Lesson Transcript Study Com
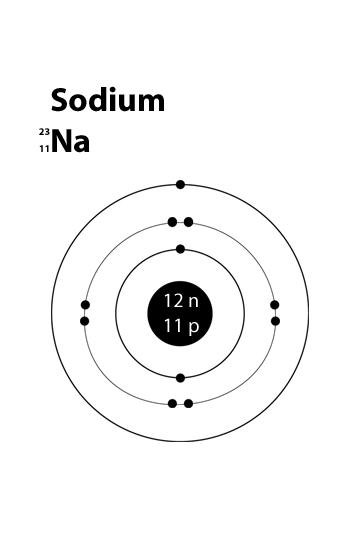



How Can I Make A Sodium Atom Model Socratic
Draw a simple atomic model for an atom of neon, Ne Answer No answers yet View Answer Topics Atoms, Molecules and Ions Composition Living by Chemistry Chapter 3 A World of Particles Section 2 Atomic Number and Atomic Mass Discussion You must be signed in to discuss Top Chemistry 101 EducatorsDraw a sketch of Bohr's model of an atom with four shells Easy View solution Explain Bohr model of atoms?I model how they will draw the atom on their activity sheet Using the colors I expect them to use, I draw the helium atom on my activity sheet as it is projected and students follow along I ask students to answer the questions below the model themselves and give them about a minute to finish, then give them permission to check with their



Rutherford Model Wikipedia



Bohr Atomic Model
Problem 19 Easy Difficulty Draw a simple atomic model for an atom of neon, Ne Answer Check back soon!And 8 for the third) And to draw the atoms you fill up the inner shells first then move on to the outer shells1 Drawing the Simplest Atoms Hydrogen and Helium The most common element in the universe is hydrogen, a gas which makes up about 99% of the universe's known mass 1 Hydrogen is the main component of stars, and a star is, by far the most massive thing in any solar system 1 That's not including "dark matter," which is beyond the scope




Bohr S Model For Hydrogen Atom




Image Result For Chlorine Atom Model 3d Project Atom Model Atom Model Project Atom
Atoms Are Building Blocks Atoms are the foundation of chemistry They are the basis for everything in the Universe As you know, matter is composed of atoms Solids are made of densely packed atoms while gases have atoms that are spread out We're going to cover basics like atomic structure and bonding between atoms · To draw a Bohr model of an atom, first find the number of protons, neutrons and electrons in the atom from its atomic weight and atomic number After that, place the neutrons and the protons in the nucleus, and draw the electrons in their designated shells Find the number of electrons, protons and neutronsPing pong balls or other small round objects of three different colors Get three times as many balls as the atomic number You will use one color for the protons, one for the neutrons and one for the electrons String;



3




Thomson Atomic Model Description Image Britannica
· 1 Democritus was not able to describe atomic model in detail On his theory, Democritus only stated that atoms are in the solid form in the void sphare We can not describe the internal structure of the atom itself We now know that Atoms consist of 3 parts which are proton, neutron and electron 2Each type of atom can be identified as a specific element on the periodic table Our drawing guide depicts lithium, the third element, because it has three electrons Scroll down for a downloadable PDF of this tutorial The atom in our drawing guide is a simplified version of the atom · Bundle the protons and neutrons together to make the nucleus and put the electrons outside the atom Note that the model gets slightly more complicated when you have more than 2 electrons (if you're trying to model as realistically as possible) because only 2



Thomson Atomic Model Plum Pudding Model Postulates Limitations




Basic Chemistry Tutorial 2 Drawing Atoms Sciencemusicvideos
Some are smaller than others! · How to Draw an Atom Step by Step Simple and Easyhow to draw an atomic bomb,how to draw an atomic model,how to draw an atom e Thanks for watching our ChannelThis week we'll make a few more models with some Atomic Cookies (page 59)




Models Of The Atom The Atom Siyavula




How To Draw An Atom Really Easy Drawing Tutorial
If playback doesn't begin shortly, try restarting your device Videos you watch may8 for the second shell;Atom drawing free on ayoqq from drawing atoms worksheet answer key , sourceayoqqorg This book provides an easy stepbystep tutorial to building the 3D model of an atom The layout can be easily worked upon



A Timeline Of Atomic Models Did You Know That The Atomic Model Has By Intlink Education Medium




Lead Atomic Structure Stock Image C013 1639 Science Photo Library
0% Home AQA GCSE Combined Science Chemistry Topic Questions 1 Atomic Structure & the Periodic Table 11 Simple Model of the AtomModels of the atom The idea of the atom as the building block of matter has developed over time What was thought of as a single particle about 1 × 10‾¹º m across is now known to be a



Drawing Atoms Montessori Muddle




Atom Diagram Universe Today



Atomic Structure



Bohr Atomic Model




What Is The Plum Pudding Atomic Model Universe Today




Models Of The Atom The Atom Siyavula




The History Of The Atom Theories And Models Compound Interest




The Early Atom Boundless Physics



Introduction To The Atom Video Khan Academy




How To Draw An Atom Really Easy Drawing Tutorial



Bohr Model Of The Atom Overview And Examples
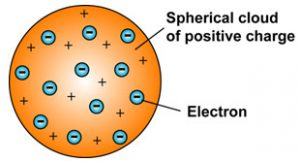



J J Thomson Model Of An Atom Class 9 Structure Of An Atom




How To Draw An Atom Youtube
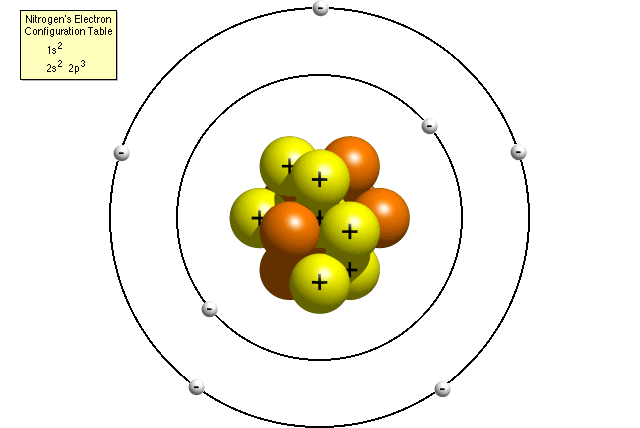



Questions And Answers How Do I Make A Model Of An Atom



Chem4kids Com Atoms Structure




Snc1p



Drawing Atoms Montessori Muddle




Basic Chemistry Tutorial 2 Drawing Atoms Sciencemusicvideos




How To Draw An Atom Step By Step Stuff Pop Culture Free Online Drawing Tutorial Added By Dawn May 7 09 2 00 11 P Atom Drawing Drawings Online Drawing
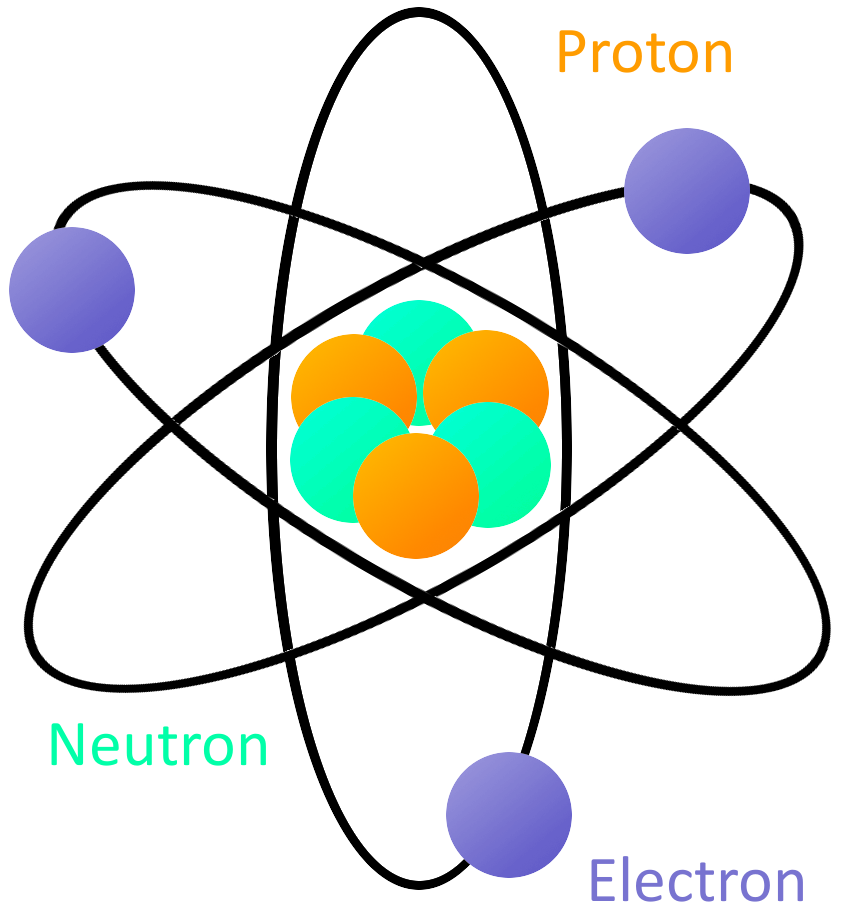



What Is Electricity Learn Sparkfun Com




Electricity Christopher Gray Engineering
:max_bytes(150000):strip_icc()/atom-drawn-by-scientist-or-student-155287893-584ee6855f9b58a8cd2fc8f1.jpg)



How To Make A Model Of An Atom




What Atomic Model Is In Use Today Socratic
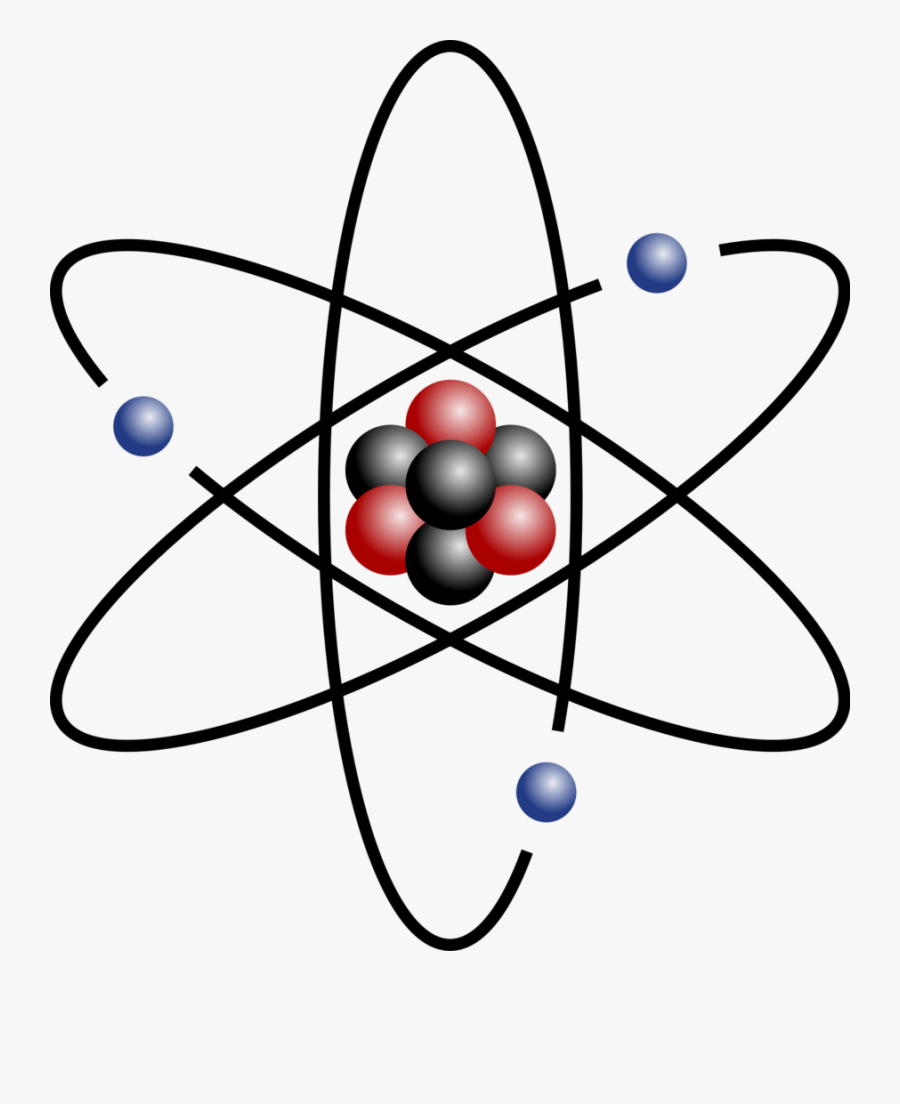



Easy To Draw Atoms Free Transparent Clipart Clipartkey




Rutherford Model Of The Atom Definition Diagram Video Lesson Transcript Study Com




Rutherford Model Of The Atom Definition Diagram Video Lesson Transcript Study Com




Oxygen Science For Kids Atom Project Atom Model Project Atom Model




Models Of The Atom The Atom Siyavula
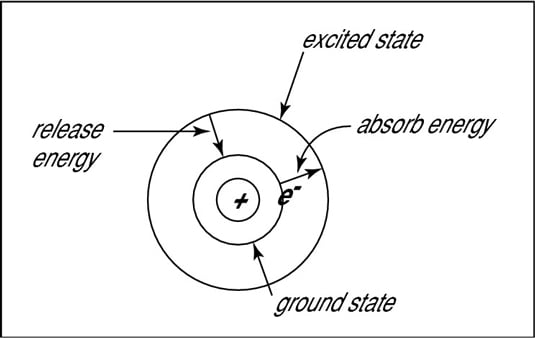



Atomic Structure The Bohr Model Dummies
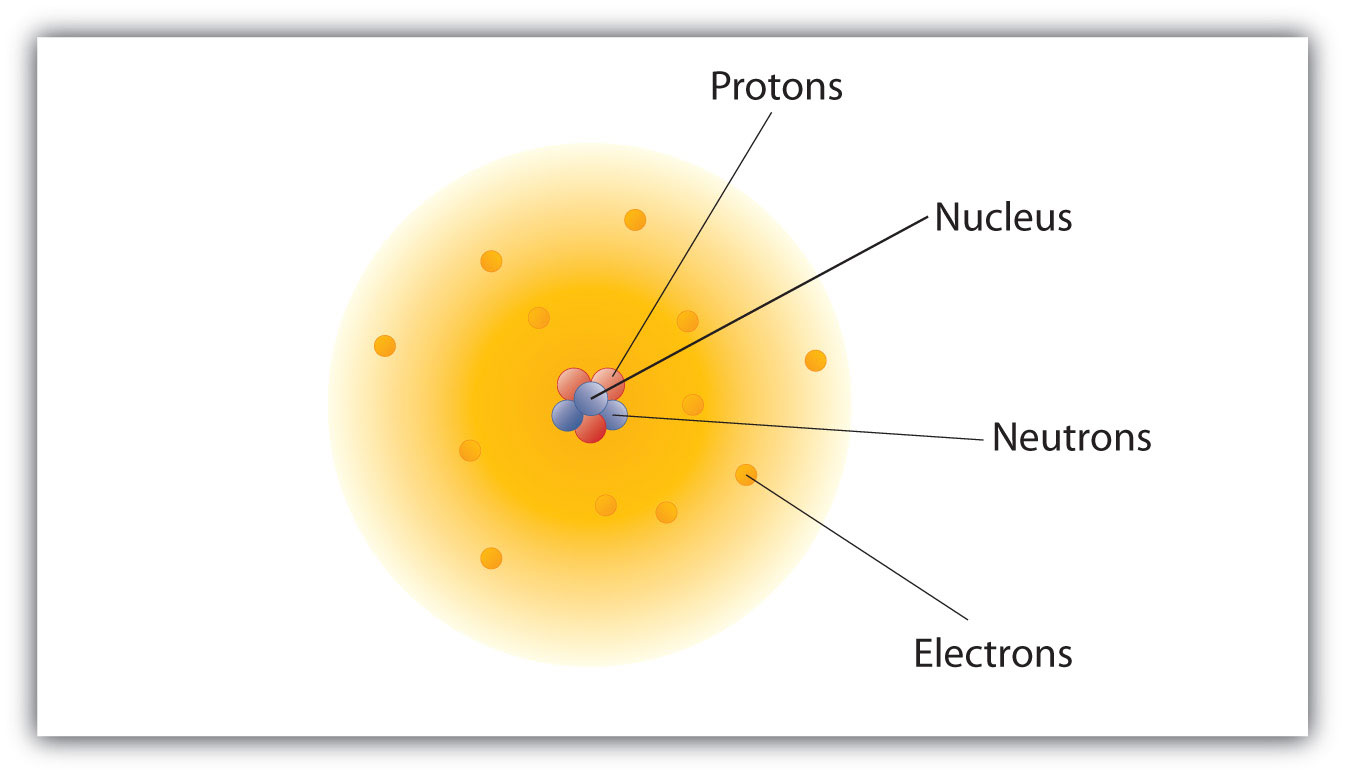



Atomic Theory




Cutting Edge Technology Atom Easy To Draw Clipart Full Size Clipart Pinclipart




How To Draw An Atom Really Easy Drawing Tutorial




Niels Bohr Boredbohr Profile Pinterest



Questions And Answers How Do I Make A Model Of An Atom




What Are The Parts Of An Atom Universe Today




What Is An Atom Live Science



What Is The Currently Accepted Model Of Atomic Structure Is There A More Recent Model Than The Bohr Or Bohr Sommerfeld That Has Been Observed By Physicists Quora




Atoms Lesson Plan A Complete Science Lesson Using The 5e Method Of Instruction Kesler Science



1
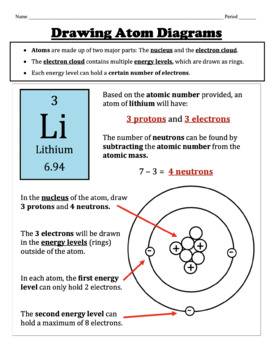



How To Draw An Atom Worksheets Teaching Resources Tpt



Atom Wikipedia




Electron Cloud Atomic Model Ck 12 Foundation
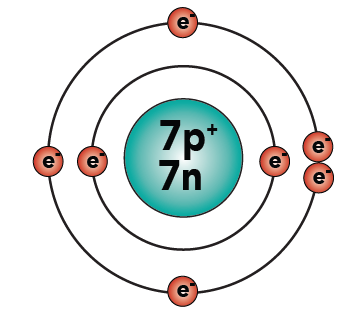



Basic Chemistry Tutorial 2 Drawing Atoms Sciencemusicvideos
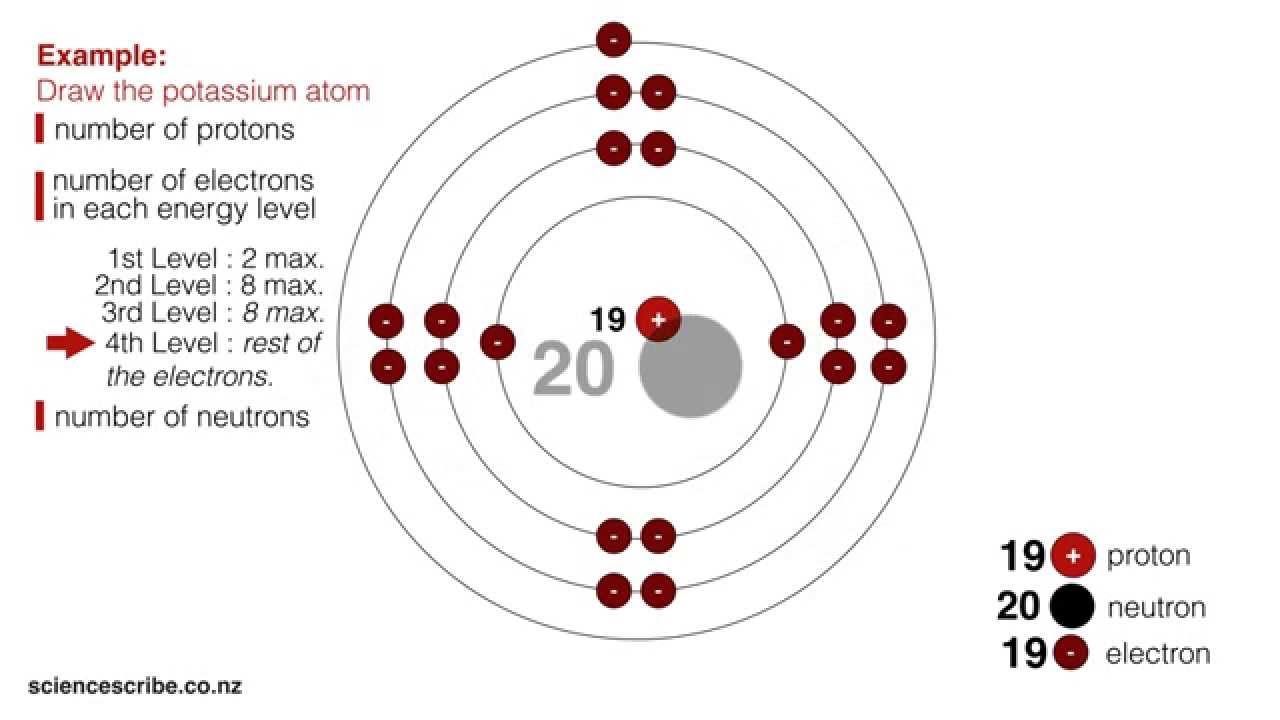



Drawing Atoms Ncea L1 Junior Science Youtube



Chem4kids Com Atoms Structure




Can You Suggest An Easy Way To Draw Atoms Tex Latex Stack Exchange
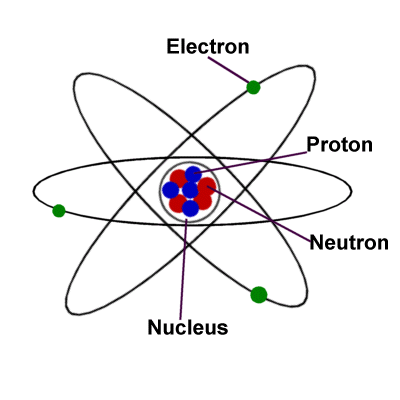



Science For Kids The Atom



Rutherford Model Wikipedia




A Timeline Of Atomic Models Did You Know That The Atomic Model Has By Intlink Education Medium
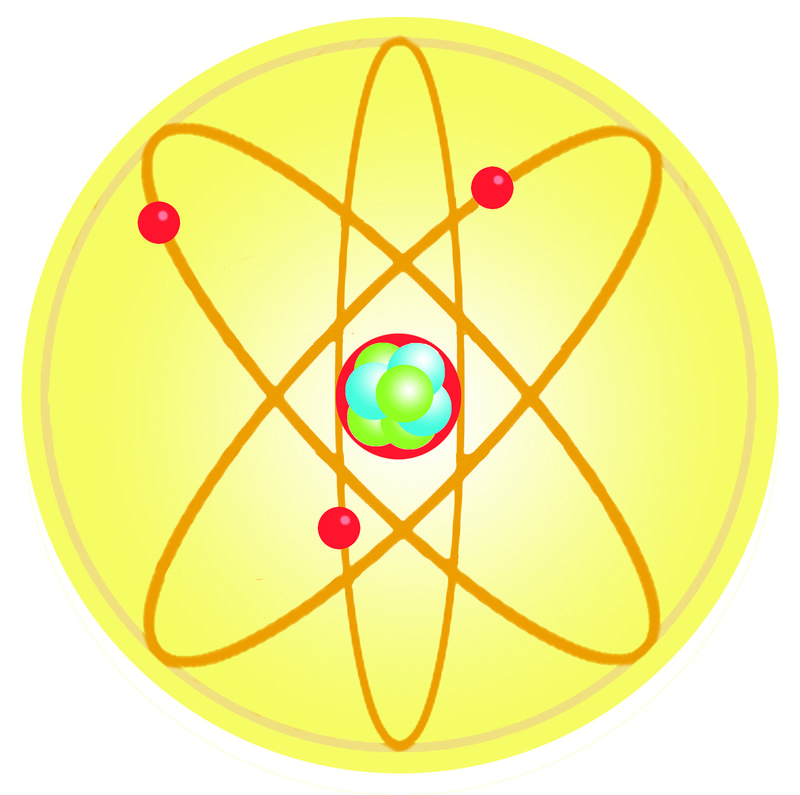



Dalton S Atomic Theory Read Chemistry Ck 12 Foundation




The Development Of The Atomic Model Wired




How To Draw Atom Step By Step Simple Easy Youtube



Difference Between Bohr And Rutherford S Atomic Models With Comparison Chart Bio Differences




How To Draw An Atom Step By Step Drawing Guide By Dawn Dragoart Com




Bohr S Model Of An Atom With Postulates And Limitations Byju S
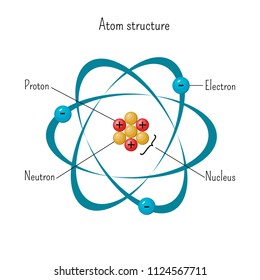



Atom Model High Res Stock Images Shutterstock



The Structure Of The Atom Boundless Chemistry




How To Draw An Atom Really Easy Drawing Tutorial
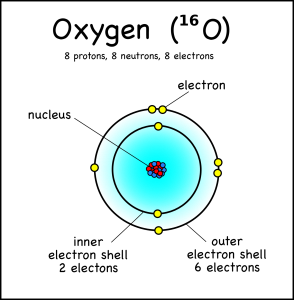



Drawing Atoms Montessori Muddle




How To Make A Small 3d Atom Model Wikihow




How To Draw Bohr Models Youtube




Bohr S Model Of An Atom With Postulates And Limitations Byju S




Question 3 Draw A Sketch Of Bohr S Model Of An Atom With Three Shells Class 9 Science Structure Brainly In
/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)



Basic Model Of The Atom Atomic Theory




Can You Suggest An Easy Way To Draw Atoms Tex Latex Stack Exchange



1




How To Draw An Atom Really Easy Drawing Tutorial



How To Draw An Atomic Structure Youtube
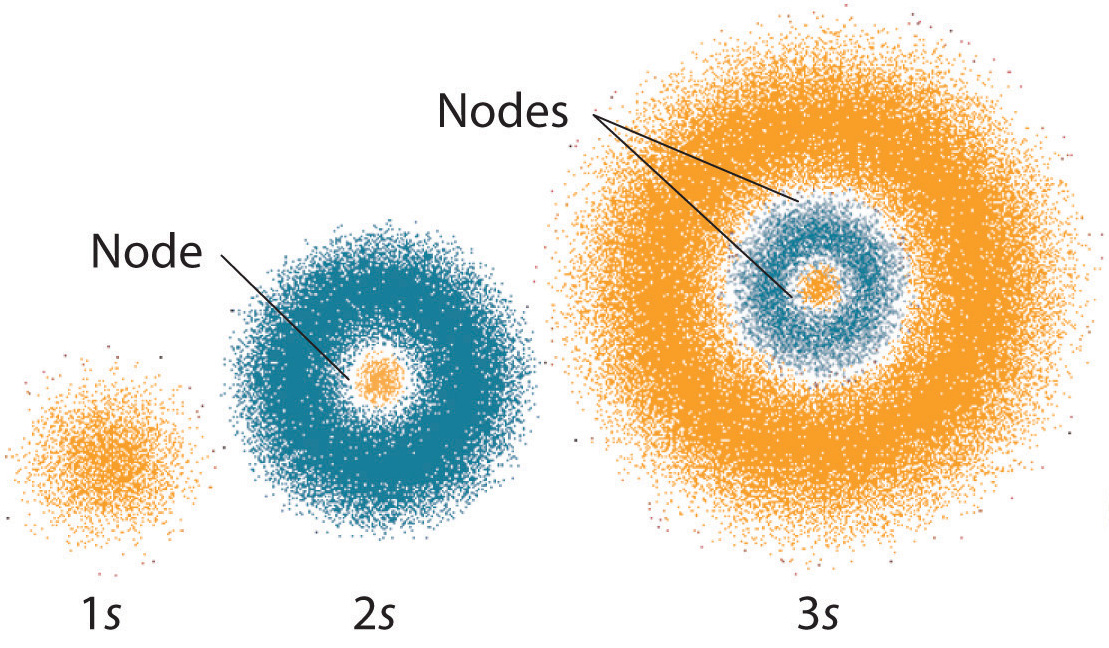



The Quantum Mechanical Model Of The Atom Article Khan Academy
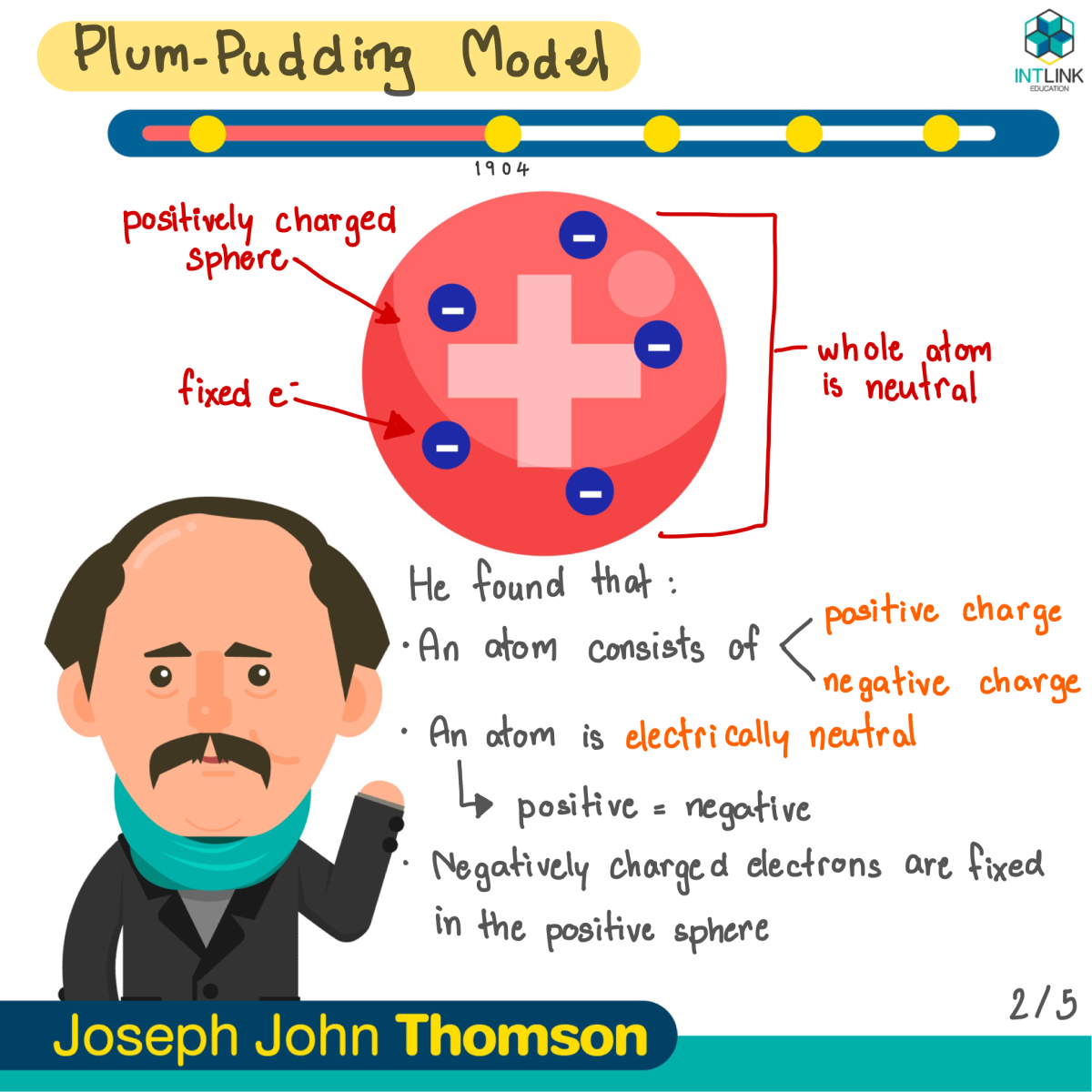



A Timeline Of Atomic Models Did You Know That The Atomic Model Has By Intlink Education Medium
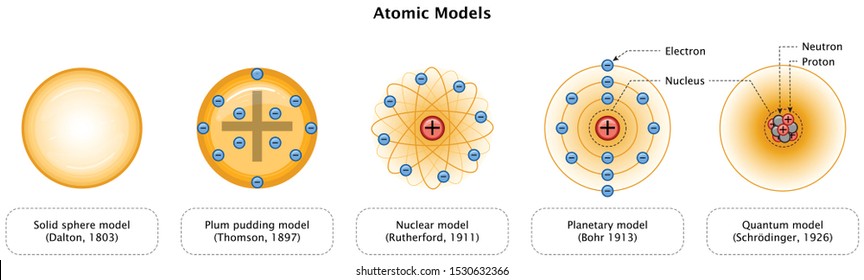



Atom Model High Res Stock Images Shutterstock



1



How To Draw The Atomic Structure Of Atoms




Atoms To Molecules Read Earth Science Science Chemistry Chemistry Education Atom Diagram




How To Draw An Atom Really Easy Drawing Tutorial
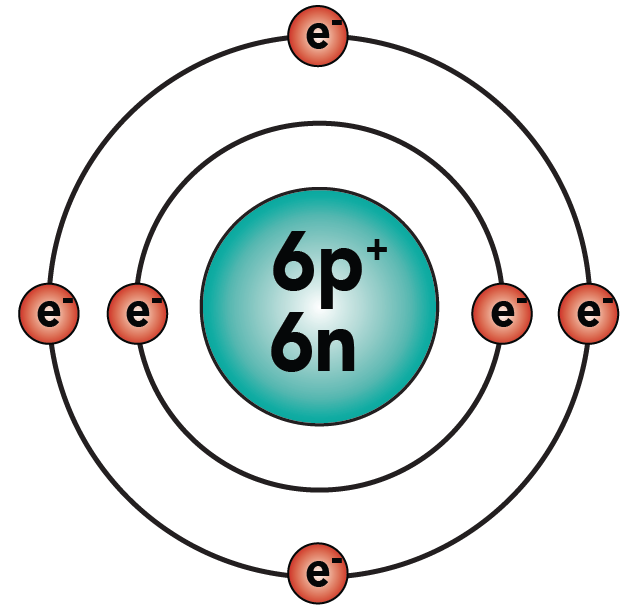



Basic Chemistry Tutorial 2 Drawing Atoms Sciencemusicvideos



0 件のコメント:
コメントを投稿